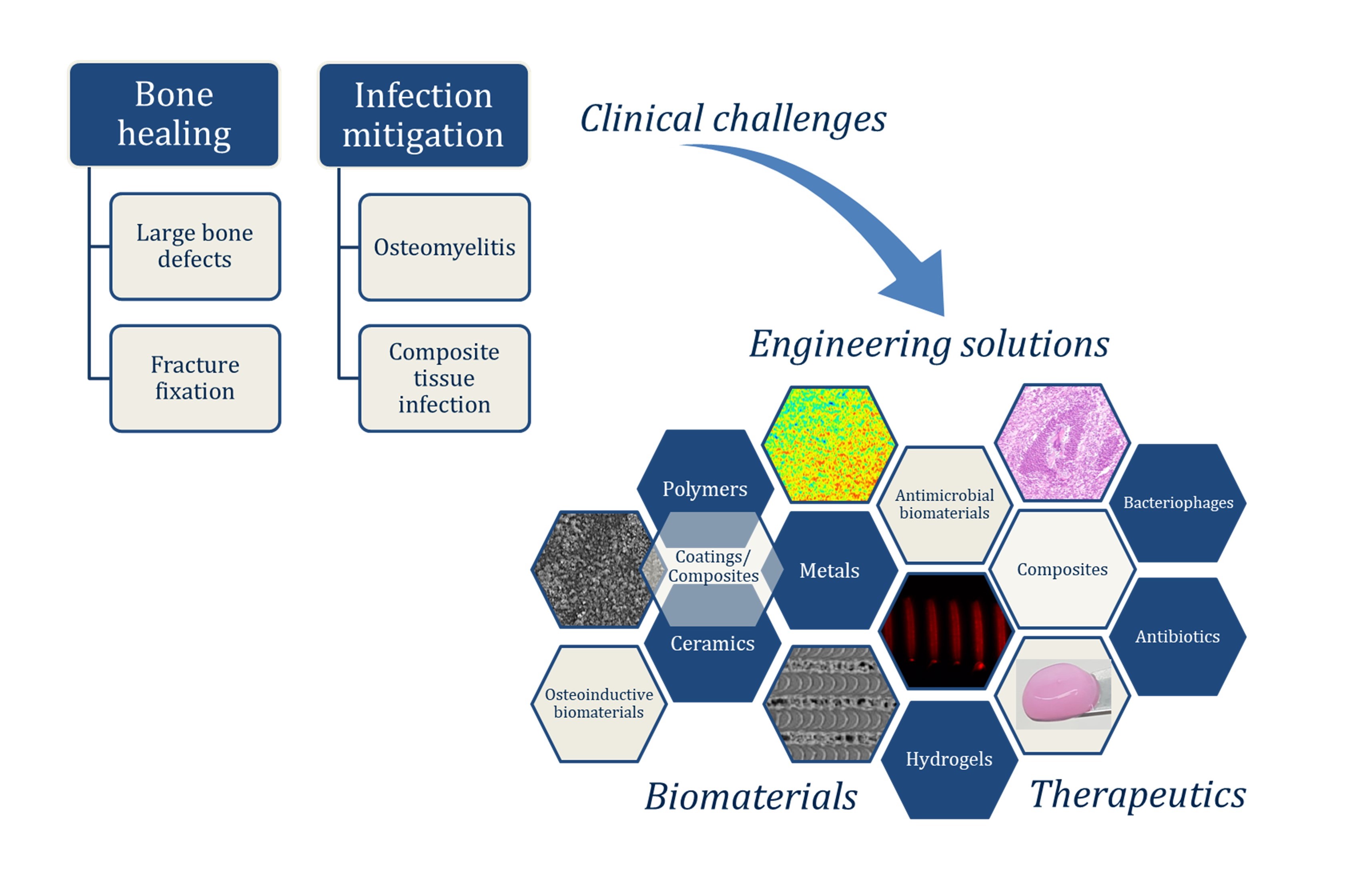
Why Infection Mitigation?
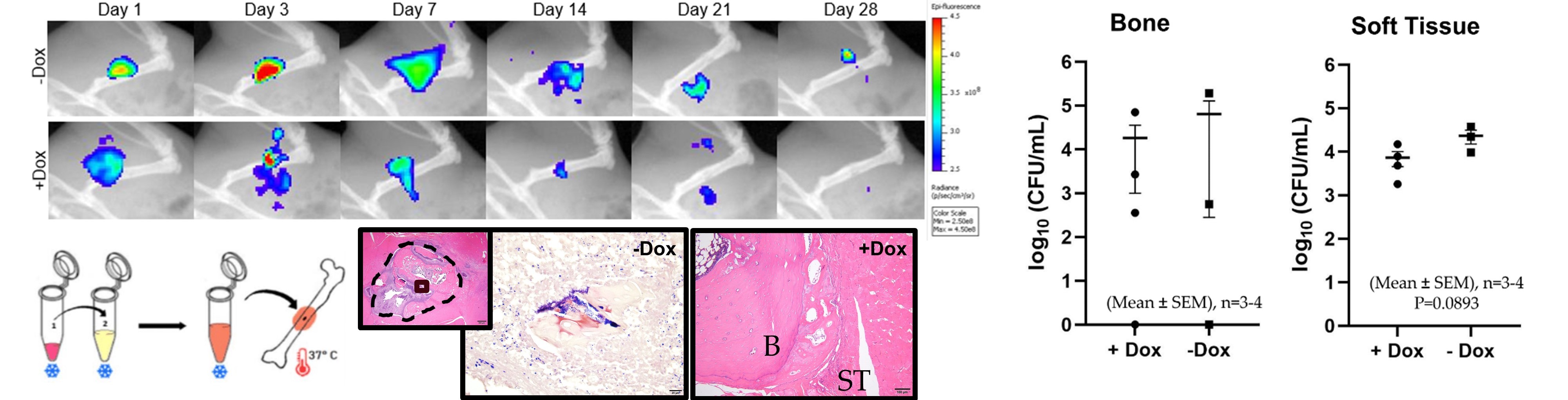
Difficulties in treating osteomyelitis, or the infection of bone, have been exacerbated by the rise of antibiotic resistant bacterial strains, particularly Staphylococcus aureus, and chronic infection remains a huge clinical burden. Development and characterization of novel antimicrobial therapeutics designed for targeted, prolonged delivery in clinically relevant scenarios will provide a new capacity to combat these most challenging, recalcitrant infections. Using a biofilm forming, green fluorescent protein (GFP) modified strain of S. aureus, we established an implant-based rat model of composite femoral and soft tissue chronic infection as a testbed for evaluating transformative biomaterial technologies. Fluorescent and radiographic images allow for longitudinal monitoring of infection in vivo.
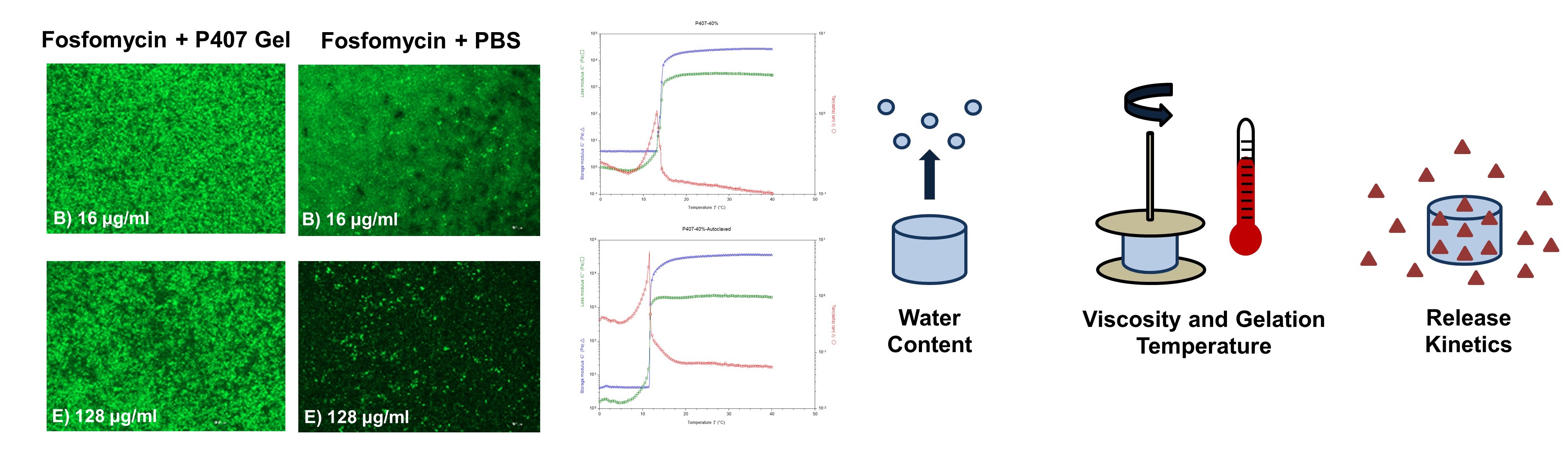
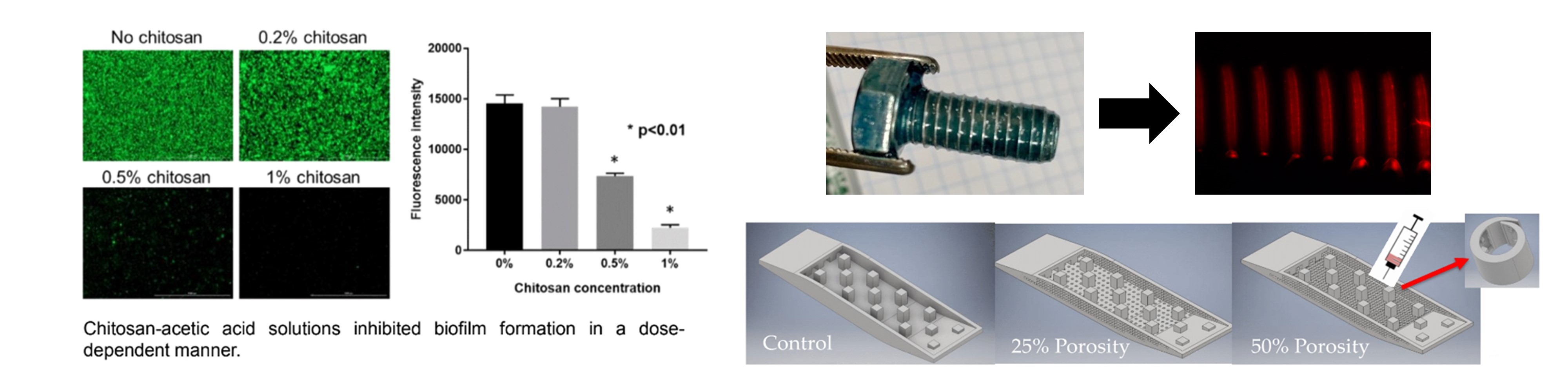
We design and fabricate hydrogel-based composite materials for enhanced delivery of antimicrobial therapeutics.
Chitosan-PCL Composite Biomaterials
The objective of this work is to evaluate the efficacy of a hybrid chitosan-poly(lactic acid) (PLA) biomaterial to deliver the antibiotic fosfomycin to the site of osteomyelitis, for treatment of bone infection. The composite material of chitosan hydrogel loaded with fosfomycin, and PLA powder loaded with fosfomycin, is expected to promote a sustained release of antibiotic into the infected bone and surrounding soft tissue. By harnessing the innate antimicrobial properties of chitosan for prolonged antimicrobial activity, the dose of antibiotic may be lowered, minimizing the risk of both host toxicity and pathogen antimicrobial resistance.
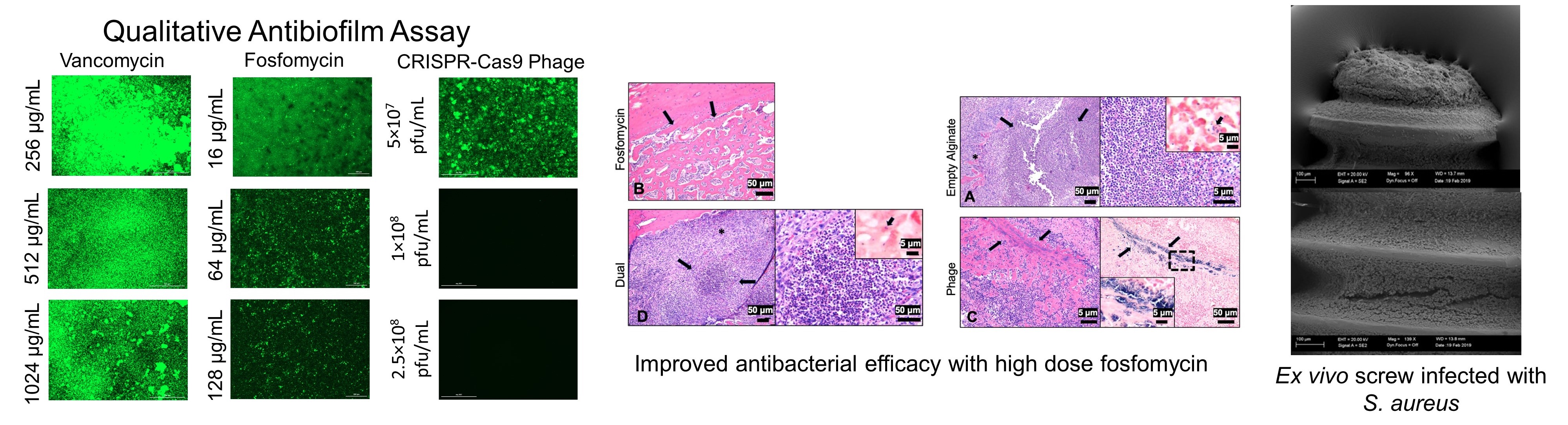
CRISPR-Cas9 Bacteriophage
As populations of antibiotic resistant bacteria continue to spread and new strains are isolated, engineering alternative therapeutics such as bacteriophage will be essential to augment the dwindling list of effective, available antibiotics. To this end, we are studying the antimicrobial efficacy of a novel CRISPR-Cas9 modified bacteriophage delivered in alginate hydrogel against S. aureus infection.