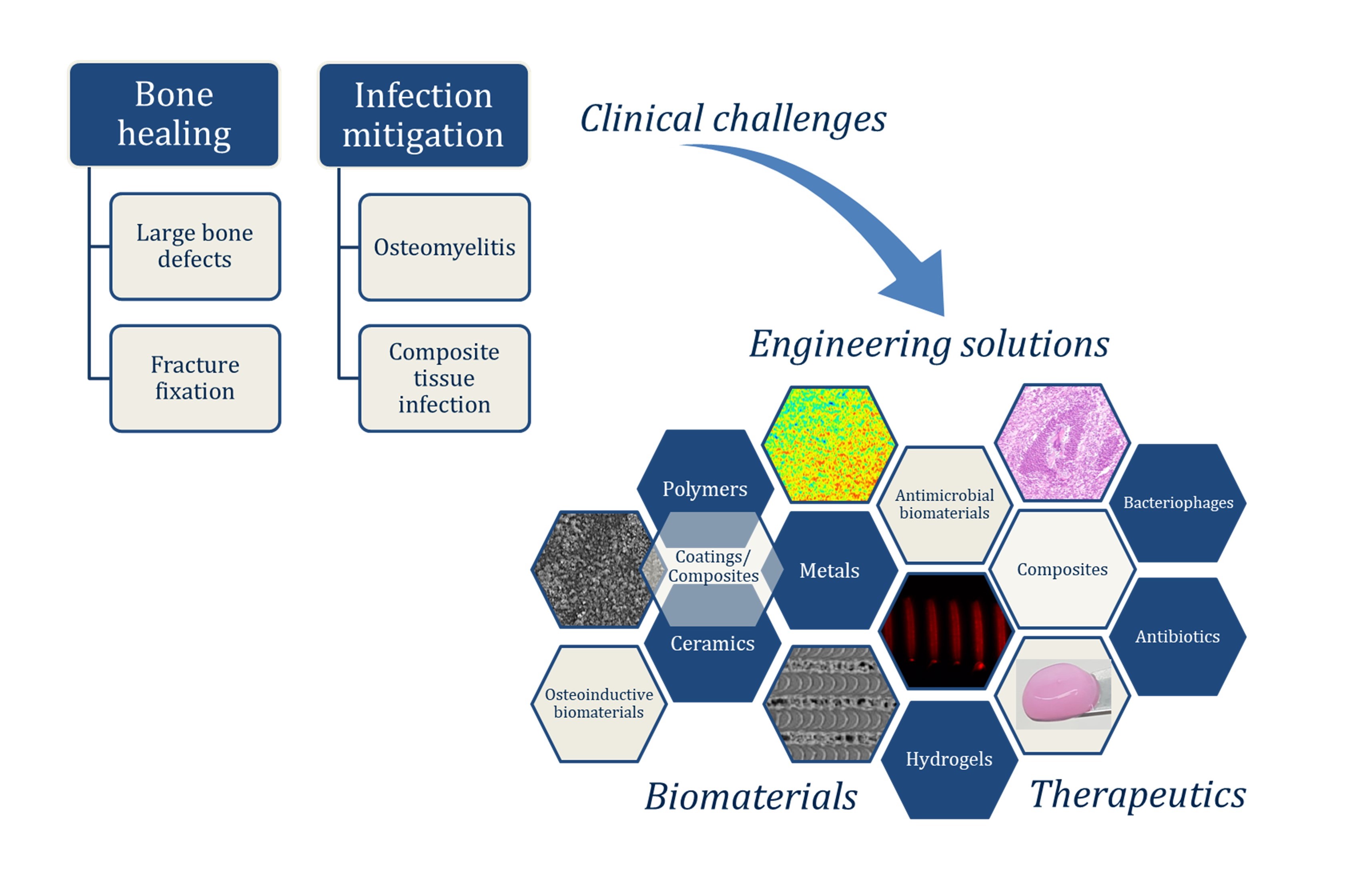
Why bone healing?
Treatment of large bone defects resulting from traumatic injury or tumor resection represents a significant challenge for orthopedic surgeons. Despite advances in therapeutics (e.g., recombinant protein technologies), the materials used for fixation of bone (to stabilize it and promote healing) are often much stronger than bone, which can lead to stress shielding, poor osseointegration, implant loosening/failure, pain, and revision surgeries for removal of the implant.
We design and fabricate customized, load-bearing polymer and metal scaffolds for improved bone healing.
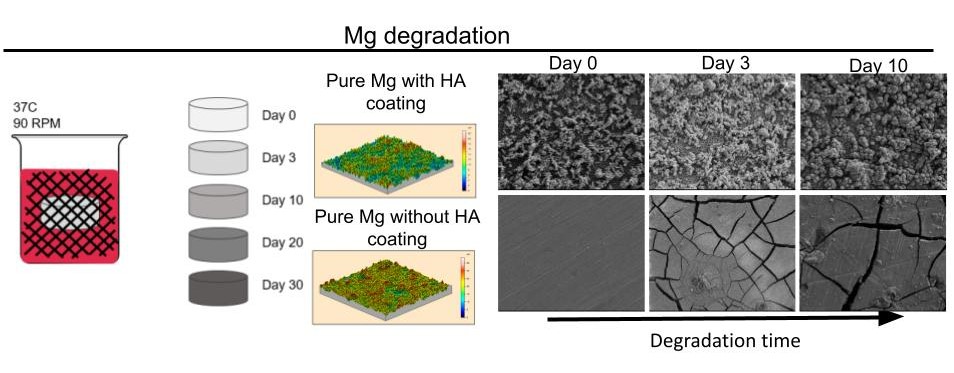
Surface Modifications
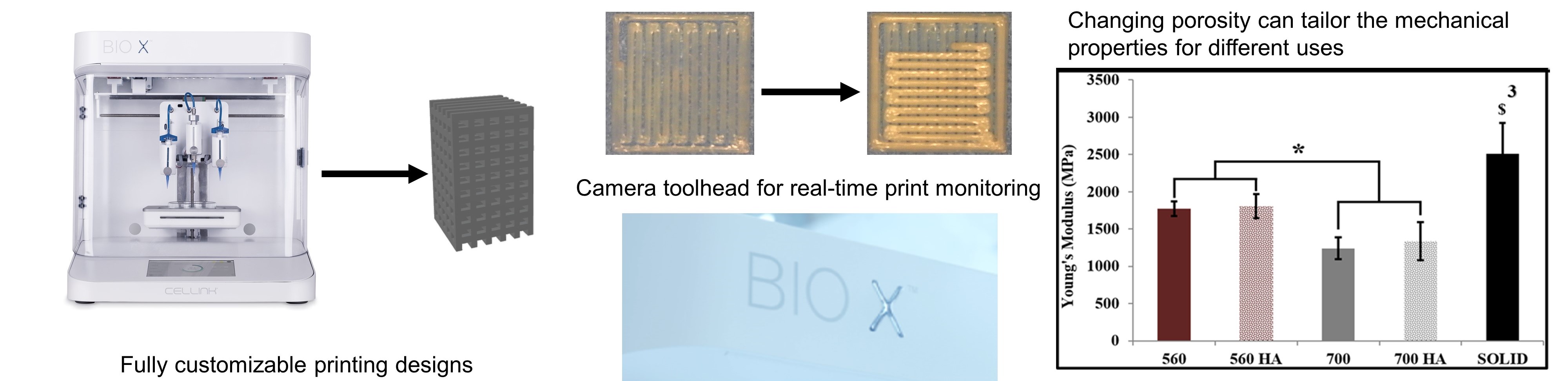
3D Printed Polymers
We are using 3D printing to create customized, load-bearing thermoplastic polymer scaffolds, and surface functionalization techniques to enhance bioceramic coatings of the polymers (e.g., polylactic acid, poly(lactic-co-glycolic acid), and poly(B-caprolactone)). Utilizing these technologies, our goal is to engineer patient-specific biomaterials that mimic both the mechanical and biological properties of native bone tissue.